Abstract
Immune thrombocytopenia (ITP) is an autoimmune disease characterized by decreased platelet (PLT) count and hemorrhagic tendency. With the development of thrombopoietin receptor agonist (TPO-RA), the prognosis of ITP who failed from first-line treatment had greatly improved. About 40-60% of patients relapse during the glucocorticoid reduction or withdrawal and require the second-line treatment. As a second-line priority recommendation, EPAG (an orally absorbable non-peptide small molecule TPO-RA) provides a sustained response in 70-80% of patients on long-term treatment for chronic ITP and 30% sustained remission rate after drug withdrawal. However, the prognostic indicators for patients who failed the initial treatment or relapsed during the treatment with EPAG are yet to be confirmed, and these may become truly refractory ITP patients in the current clinical practice. To date, only a limited number of studies have focused on how to improve the efficiency of EPAG-resistant ITP, including the novel drugs (such as fostamatinib, efgartigimod, etc.). According to the Chinese guidelines on the diagnosis and management of adult immune thrombocytopenia, immunosuppressive therapy (IST), such as cyclosporine A (CsA) and azathioprine, are recommended if patients failed the second-line treatment. However, the efficacy of mono-IST-therapy has a prolonged onset time (6-8 weeks for CsA and 7 weeks for azathioprine) and a high incidence of side effects. Currently, a few studies have reported the combination effect of EPAG with IST for refractory ITP. The total response of EAPG combined with azathioprine for refractory ITP patients was significantly higher than azathioprine alone (90.32% vs. 73.33%), but the incidence of side effects was also increased significantly, especially the hepatotoxicity (~25%). Thus, a safe and rapid response combination treatment should be explored, especially for EPAG-resistant ITP. Herein, we conducted a study to explore the efficacy and safety of EPAG combined with low-dose CsA in the management of multiline failed ITP patients who did not respond to prior EPAG single treatment.
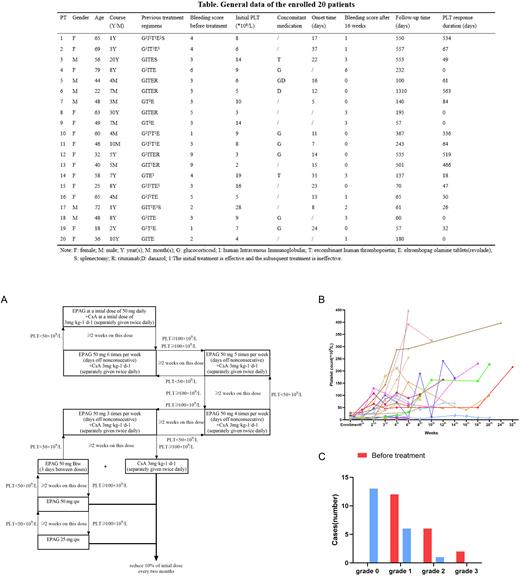
A total of 20 ITP patients who failed multiline therapies, including prior administration of 75 mg EPAG for at least 30 days were enrolled at The First Affiliated Hospital of Zhejiang Chinese Medical University (Hangzhou, China) from January 2018 to March 2022 (showed in Table). The median PLT count before treatment was 8 (2-28) ×109/L, and was associated with varying degrees of bleeding and a bleeding score of 3 (1-9). All patients continued with 50mg EPAG and received additional low-dose CsA (with a target concentration 75-120 ng/mL). 75% (15/20) patients responded to the combination treatment, and the median response time was 15 days. 73.3% (11/15) responsive patients achieved complete response (CR) during the follow-up period, 1 patient relapsed from CR due to self-reduction of EPAG and regained the response after restarting EPAG. The dose titration protocol of EPAG and CsA was shown in Figure A. No relapse was observed during the follow-up period in 15 patients with a curative effect. The median duration of platelet sustained response was 64 (18-563) days (Fig B). All patients had bleeding symptoms before treatment, and decreased to 35% (7/20) of patients after 16 weeks treatment. Among them, 85.7% (6/7) of patients presented Grade 1 bleeding (petechiae), and only 1/7 cases had Grade 2 bleeding (oral bleeding) (Fig C). During a median follow-up of 187.5 days, 70% (7/10) patients stopped or decreased the concomitant medication. Regarding side effects, only one patient suffered from venous embolic cerebral infarction, which was relieved after symptomatic treatment, and no other severe drug-related side effects were noticed during the study (total events 20%).
In summary, the combination therapy of EPAG and CsA is beneficial for the quick onset and long-term efficacy maintenance, which could be a novel and effective method for the ITP patients with prior EPAG and multiline treatment failure.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal